the electron dot structure for ascl3 shows|AsCl3 Lewis Structure : Bacolod In the AsCl 3 Lewis structure, there are three single bonds around the arsenic atom, with three chlorine atoms attached to it. Each chlorine atom has three .
Just last year, we saw record-breaking high ocean temperatures and sea level is projected to rise up to 12 inches by 2050. You can make a difference by supporting Ocean Conservancy's work toward policy, research and solutions to our ocean's greatest threat. Give today to support the critical work to protect our ocean.Hello everybody, here we are today with Wordalot, new exciting quiz for Android. This is a brand new game developed by MAG Interactive who have also developed Wordbrain, Wordbrain Themes and Ruzzle. This game is not like the Wordbrain game which all you need to do was to guess what words where shown in each level and slide the finger .
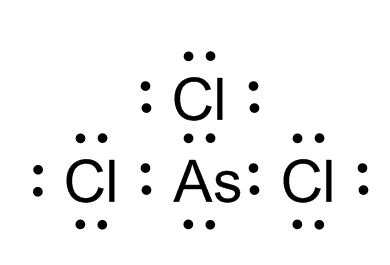
the electron dot structure for ascl3 shows,A step-by-step explanation of how to draw the AsCl3 Lewis Dot Structure ( Arsenic trichloride).For the AsCl3 structure use the periodic table to find the tot.

Question: QUESTION 5 The electron dot structure for AsCl3 shows three single bonds and one lone pair. two single bonds, one double bond, and 9 lone pairs. one single bond, two double bonds, and 8 lone .
The electron dot structure of the AsCl3 molecule is also known as the AsCl3 Lewis structure. It determines the number of outermost valence electrons as well as the . Total valence electrons in AsCl3 molecule. → Valence electrons given by arsenic atom: Arsenic is a group 15 element on the periodic table. [1] Hence the valence electrons present in arsenic is 5. .

For the AsCl 3 Lewis structure there are a total of 26 valence electrons available. AsCl3 Lewis Structure: How to Draw the Lewis Dot Structure for AsCl3. Share. Watch on. . In the AsCl 3 Lewis structure, there are three single bonds around the arsenic atom, with three chlorine atoms attached to it. Each chlorine atom has three . Chemistry learning made easy.This tutorial will help you deal with the lewis structure and moleculargeometry for arsenic trichloride (AsCl3).
Provide the most probable Lewis structure for compound AsCl3. Skip to main content. General Chemistry Start typing, then use the up and down arrows to select an option .
A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom . The Lewis dot symbol for the calcium ion is. A) 2+ B) Ca C) 2+ D) Ca2+ E) Ca. 2. The electron dot structure for AsCl3 molecule shows. A) a total of 84 electron dots. B) three single bonds and 10 lone pairs. C) two single bonds, one double bond, and 9 lone pairs. D) one single bond, two double bonds, and 8 lone pairs. E) three single .The electron dot structure for {eq}AsCl_3 {/eq} shows a total of 84 electron dots. Electron Dot Structure: Electron dot structure is simply Lewis structure which shows bonding present between the atoms of molecule.There remains 20 VE after connecting the three Cl atoms to the central atom. These 20 electrons will be distributed to complete the octet of each atom. Each Cl atom needs 6 and As needs 2 more. We can see that the electron dot structure for AsCl3 shows three single bonds and 10 lone pairs. Therefore, the statement is true.
The -2 charge means that there are 2 extra electrons. Total: 4 + (3 × 6) + 2 = 24 electrons. The final answer MUST have this number of electrons‼! Step 2) Attach the atoms to each other using single bonds (“draw the skeleton structure”) Step 3) Add electrons to all outer atoms (except H) to complete their octets.The Lewis structure for AsCl3 shows two single bonds, one double bond and 9 lone pairs O three single bonds and 10 lone pairs O three single bonds and one lone pair one single bond, two double bonds and 8 lone pairs. World of Chemistry, 3rd edition. 3rd Edition. ISBN: 9781133109655. Author: Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste. Here’s how you can easily draw the AsCl 3 Lewis structure step by step: #1 Draw a rough skeleton structure. #2 Mention lone pairs on the atoms. #3 If needed, mention formal charges on the atoms. Now, let’s take .the electron dot structure for ascl3 shows The answer is B) trigonal pyramidal. To determine the molecular geometry of arsenic trichloride, AsCl_3, you must take a look at its Lewis structure. One arsenic trichloride molecule will have a total of 26 valence electrons - 5 from the arsenic atom and 7 from each of the three chlorine atoms. The arsenic atom will be bonded to the three .
Provide the most probable Lewis structure for compound AsCl3. Skip to main content. General Chemistry Start typing, then use the up and down arrows to select an option from the list. . Electron Capture & Positron Emission (0) Band of Stability: Overview (0) . Bonding & Molecular Structure Lewis Dot Structures: .AsCl3 Lewis Structure A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above .
the electron dot structure for ascl3 shows AsCl3 Lewis Structure What is the formal charge of sulfur in the best Lewis structure for the SCN (thiocyanate) ion? 0. The electron dot structure for AsCl3 shows. three single bonds and 10 lone pairs. Which of these choices is a correct lewis structure for ozone, O3? (6 dots) ---- (2dots) _-_-_-_-_-_ (4 dots) The shape of the SF4 molecule is.The electron dot structure for AsCl_3 shows a total of 84 electron dots. three single bonds and 10 lone pairs. two single bonds, one double bond, and 9 lone pairs. one single bond, two double bonds, a; The electron-dot structure for the species N H 4 O + contains (a) 1 double bond and 4 single bonds. (b) 5 single bonds and 3 lone pairs.The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. (It does not matter what . The electron dot structure for AsCl3 shows. A. a total of 84 electron dots. B. three songle bonds and 10 lone pairs. C. two single bonds, one double bond, 9 lone pairs. D. one single bond, two double bonds, 8 lone pairs.The lewis dot structure for AsCl3 shows? Three single bonds and 10 lone pairs. . Distorted tetrahedron. What is the shape of SO4 2-Tetrahedral. The lewis dot structure for ClO3- should show.single bonds,.double bonds, and.lone pairs. 3,0,10. How many lone electron pairs does N2 have? 2. What is the shape of PH3? Trigonal pyramidal. What .The electron dot structure for AsCl3 shows. The electron configuration of a ground-state vanadium atom is. . The electron dot formula for O2 shows. Choose matching definition. a single covalent bond. a double covalent bond. hydrogen bonds. an ionic bond. Don't know? 12 of 20. Term.The Lewis structure for AsClz has a total of 10 lone pairs. The central atom (As) has 4 electron groups around it. The Lewis structure for AsCl3 has 3 bonding pairs. The electron geometry around the central atom, As is trigonal pyramidal. The central atom, As has one lone pair. ΟΟ none of the above. A hydrogen atom is shown as H⋅ H ⋅ because of its one valence electron. The structures of molecules that are held together by covalent bonds can be diagrammed by Lewis electron-dot structures. The hydrogen molecule is shown in the figure below. Figure 9.5.2 9.5. 2: On the left is a single hydrogen atom with one electron.ÐÏ à¡± á> þÿ y { þÿÿÿx .
the electron dot structure for ascl3 shows|AsCl3 Lewis Structure
PH0 · Solved QUESTION 5 The electron dot structure for
PH1 · SOLVED: QUESTION 5 The electron dot structure for AsCl3 show
PH2 · Provide the most probable Lewis structure for compound AsCl3
PH3 · Lewis Structure of AsCl3 (With 6 Simple Steps to Draw!)
PH4 · How to draw AsCl3 Lewis Structure?
PH5 · AsCl3 Molecular Shape + Lewis Dot Structure
PH6 · AsCl3 Lewis structure
PH7 · AsCl3 Lewis Structure: How to Draw the Lewis Dot Structure for
PH8 · AsCl3 Lewis Structure: How to Draw the Lewis Dot Structure
PH9 · AsCl3 Lewis Structure in 6 Steps (With Images)
PH10 · AsCl3 Lewis Structure
PH11 · 9.2: Lewis Electron Dot Diagrams